
| ĂűłĆ | Transmembrane protease serine 2 |
| ´ż¶Č | >90% |
| ËŢÖ÷ϸ°ű | HEK293 |
| ÄÚ¶ľËŘ | <10 EU/mg |
| ĐňÁĐ | MALNSGSPPAIGPYYENHGYQPENPYPAQPTVVPTVYEVHPAQYYPSPVPQYAPRVLTQASNPVVCTQPKSPSGTVCTSKTKKALCITLTLGTFLVGAALAAGLLWKFMGSKCSNSGIECDSSGTCINPSNWCDGVSHCPGGEDENRCVRLYGPNFILQVYSSQRKSWHPVCQDDWNENYGRAACRDMGYKNNFYSSQGIVDDSGSTSFMKLNTSAGNVDIYKKLYHSDACSSKAVVSLRCIACGVNLNSSRQSRIVGGESALPGAWPWQVSLHVQNVHVCGGSIITPEWIVTAAHCVEKPLNNPWHWTAFAGILRQSFMFYGAGYQVEKVISHPNYDSKTKNNDIALMKLQKPLTFNDLVKPVCLPNPGMMLQPEQLCWISGWGATEEKGKTSEVLNAAKVLLIETQRCNSRYVYDNLITPAMICAGFLQGNVDSCQGDSGGPLVTSKNNIWWLIGDTSWGSGCAKAYRPGVYGNVMVFTDWIYRQMRADG |
| »ůŇňĂű | TMPRSS2 |
| Uniprot ID | O15393 |
| ÎďÖÖ | Homo sapiens(Human) |
| ˛úĆ·ĐÔ×´ | Liquid |
| »şłĺŇş | 1ˇÁPBSŁ¬pH7.4 |
| ´˘´ć·˝Ę˝ | -80 ˇć packaging and storage to avoid repeated freezing and thawing. |
| SDS-PAGE &WB |
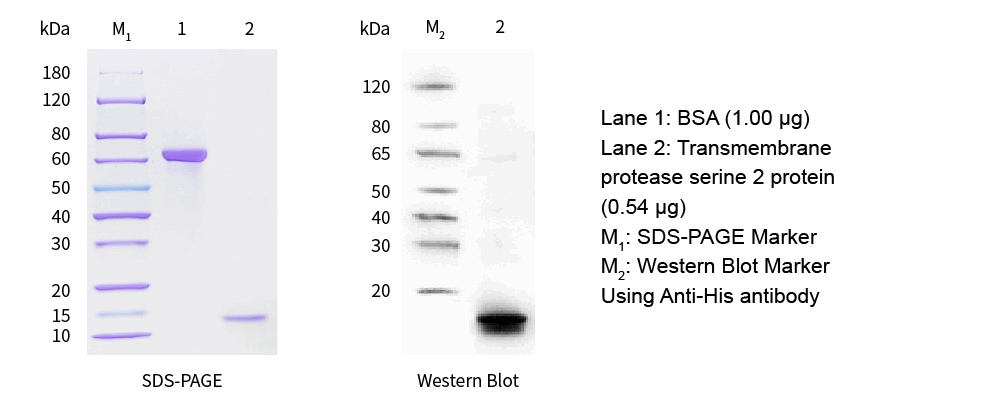
|
(Microbial infection) Facilitates human coronaviruses SARS-CoV and SARS-CoV-2 infections via two independent mechanisms, proteolytic cleavage of ACE2 receptor which promotes viral uptake, and cleavage of coronavirus spike glycoproteins which activates the glycoprotein for host cell entry (PubMed:24227843, PubMed:32142651, PubMed:32404436, PubMed:33051876, PubMed:34159616). The cleavage of SARS-COV2 spike glycoprotein occurs between the S2 and S2' site (PubMed:32703818). Upon SARS-CoV-2 infection, increases syncytia formation by accelerating the fusion process (PubMed:33051876, PubMed:34159616). Proteolytically cleaves and activates the spike glycoproteins of human coronavirus 229E (HCoV-229E) and human coronavirus EMC (HCoV-EMC) and the fusion glycoproteins F0 of Sendai virus (SeV), human metapneumovirus (HMPV), human parainfluenza 1, 2, 3, 4a and 4b viruses (HPIV). Essential for spread and pathogenesis of influenza A virus (strains H1N1, H3N2 and H7N9); involved in proteolytic cleavage and activation of hemagglutinin (HA) protein which is essential for viral infectivity.


