
| ĂűłĆ | Cytoglobin Protein |
| ´ż¶Č | >85% |
| ËŢÖ÷ϸ°ű | HEK293 |
| ĐňÁĐ | MEKVPGEMEIERRERSEELSEAERKAVQAMWARLYANCEDVGVAILVRFFVNFPSAKQYFSQFKHMEDPLEMERSPQLRKHACRVMGALNTVVENLHDPDKVSSVLALVGKAHALKHKVEPVYFKILSGVILEVVAEEFASDFPPETQRAWAKLRGLIYSHVTAAYKEVGWVQQVPNATTPPATLPSSGP |
| »ůŇňĂű | CYGB |
| Uniprot ID | Q8WWM9 |
| ÎďÖÖ | Homo sapiens(Human) |
| ˛úĆ·ĐÔ×´ | Liquid |
| »şłĺŇş | 1ˇÁPBSŁ¬pH7.4 |
| ´˘´ć·˝Ę˝ | -80 ˇć packaging and storage to avoid repeated freezing and thawing. |
| SDS-PAGE &WB |
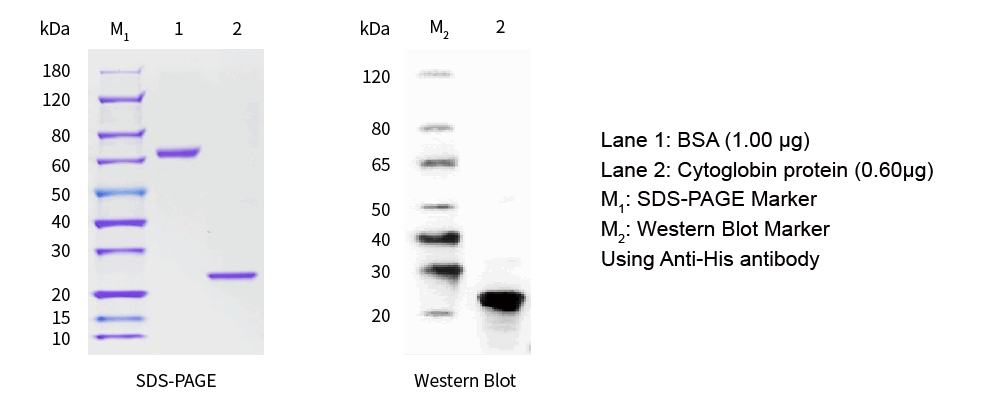
|
Probable multifunctional globin with a hexacoordinated heme iron required for the catalysis of various reactions depending on redox condition of the cell as well as oxygen availability (PubMed:11893755, PubMed:12359339, PubMed:15165856, PubMed:19147491, PubMed:20511233, PubMed:28393874, PubMed:28671819, PubMed:29128400, PubMed:33576020, PubMed:34930834). Has a nitric oxide dioxygenase (NOD) activity and is most probably involved in cell-mediated and oxygen-dependent nitric oxide consumption (PubMed:19147491, PubMed:20511233, PubMed:28393874, PubMed:28671819). By scavenging this second messenger may regulate several biological processes including endothelium-mediated vasodilation and vascular tone (PubMed:19147491, PubMed:28393874). Under normoxic conditions functions as a nitric oxide dioxygenase (NOD) but under hypoxic conditions the globin may switch its function to that of a nitrite (NO2) reductase (NiR), generating nitric oxide (PubMed:29128400). Could also have peroxidase and superoxide dismutase activities, detoxifying reactive oxygen species and protecting cells against oxidative stress (PubMed:12359339, PubMed:33576020, PubMed:34930834). Also binds dioxygen with low affinity and could function as an oxygen sensor but has probably no function as a respiratory oxygen carrier (PubMed:11893755, PubMed:15299006, PubMed:20553503).


