
| ĂűłĆ | Proto-oncogene tyrosine-protein kinase Src |
| ´ż¶Č | >90% |
| ËŢÖ÷ϸ°ű | HEK293 |
| ĐňÁĐ | MGSNKSKPKDASQRRRSLEPAENVHGAGGGAFPASQTPSKPASADGHRGPSAAFAPAAAEPKLFGGFNSSDTVTSPQRAGPLAGGVTTFVALYDYESRTETDLSFKKGERLQIVNNTEGDWWLAHSLSTGQTGYIPSNYVAPSDSIQAEEWYFGKITRRESERLLLNAENPRGTFLVRESETTKGAYCLSVSDFDNAKGLNVKHYKIRKLDSGGFYITSRTQFNSLQQLVAYYSKHADGLCHRLTTVCPTSKPQTQGLAKDAWEIPRESLRLEVKLGQGCFGEVWMGTWNGTTRVAIKTLKPGTMSPEAFLQEAQVMKKLRHEKLVQLYAVVSEEPIYIVTEYMSKGSLLDFLKGETGKYLRLPQLVDMAAQIASGMAYVERMNYVHRDLRAANILVGENLVCKVADFGLARLIEDNEYTARQGAKFPIKWTAPEAALYGRFTIKSDVWSFGILLTELTTKGRVPYPGMVNREVLDQVERGYRMPCPPECPESLHDLMCQCWRKEPEERPTFEYLQAFLEDYFTSTEPQYQPGENL |
| »ůŇňĂű | SRC |
| Uniprot ID | P12931 |
| ÎďÖÖ | Homo sapiens(Human) |
| ˛úĆ·ĐÔ×´ | Liquid |
| »şłĺŇş | 1ˇÁPBSŁ¬pH7.4 |
| ´˘´ć·˝Ę˝ | -80 ˇć packaging and storage to avoid repeated freezing and thawing. |
| SDS-PAGE &WB |
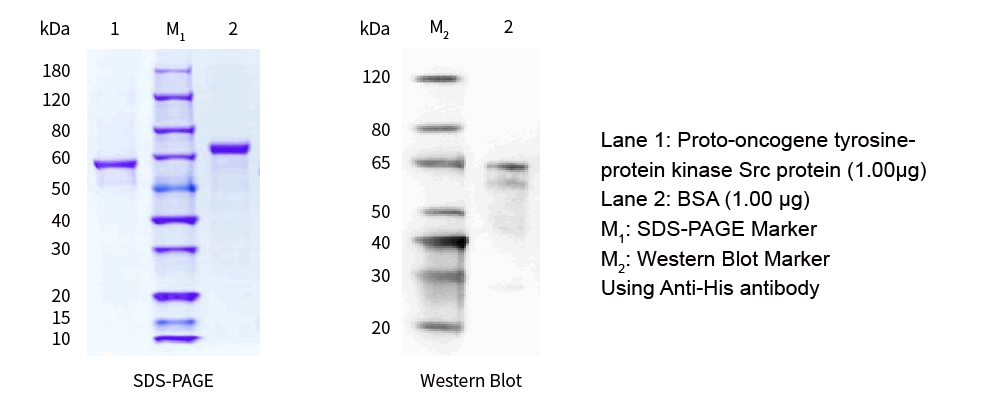
|
Non-receptor protein tyrosine kinase which shows higher basal kinase activity than isoform 1, possibly due to weakened intramolecular interactions which enhance autophosphorylation of Tyr-419 and subsequent activation (By similarity). The SH3 domain shows reduced affinity with the linker sequence between the SH2 and kinase domains which may account for the increased basal activity (By similarity). Displays altered substrate specificity compared to isoform 1, showing weak affinity for synaptophysin and for peptide substrates containing class I or class II SH3 domain-binding motifs (By similarity). Plays a role in neurite elongation (By similarity).


